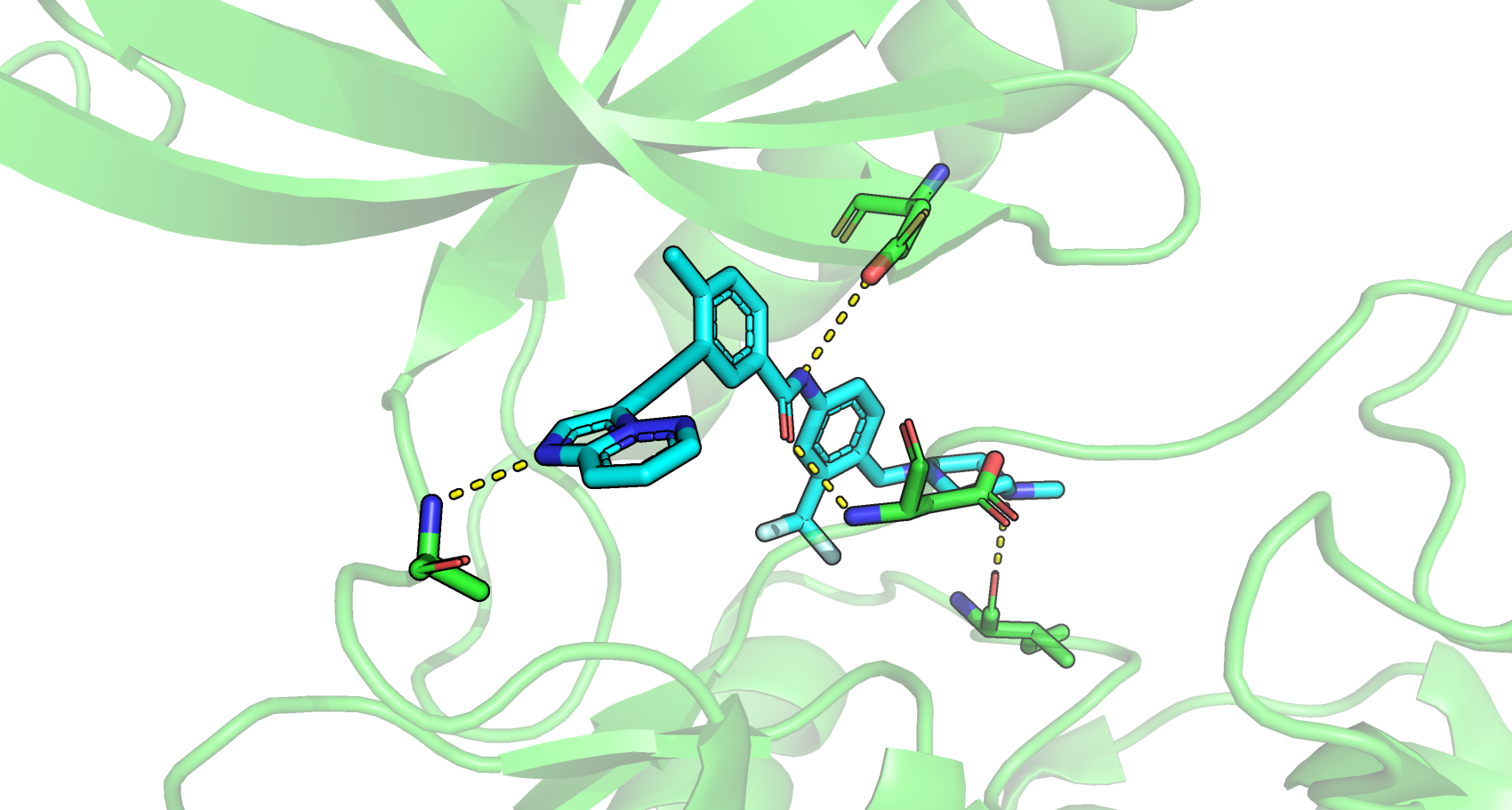
氢键作用在蛋白质-配体结合起到非常重要的稳固作用。在此记录下通常氢键形成的条件。
2.5 Å <= 键长 <=3.5 Å
120°<= 键角 <=180°
解释一
COMPUTATIONAL TECHNIQUES
Hydrogen bonds are found in protein crystallography indirectly. If a proper hydrogen bond acceptor–donor pair is within the correct distance, the bond is taken to be a hydrogen bond. This distance is generally considered to be from 2.7 to 3.3 Å, with 3.0 Å being the most common value for protein and water hydrogen bonds. The angle the bond forms is also important in determining the strength of the hydrogen bond. The closer the hydrogen bond is to correct geometry, the stronger the bond. Hydrogen bonds often occur in networks—frequently with water mediating. Water is especially facile at hydrogen bonding because it is both an acceptor and a donor. Histidines can have various protonation states, and an analysis of the hydrogen bonds can allow a determination of the most likely protonation state according to whether hydrogen is bonded to a donor or to a acceptor. In a similar manner, the orientations of histidines, threonine, glutamine, and asparagine are ambiguous in protein maps where the slight density difference between a carbon, oxygen, or nitrogen atom cannot be safely distinguished. An analysis of hydrogen bonding can give important clues in determining the correct orientation of an ambiguous side chain.
解释二
The Role of Functional Groups in Drug–Receptor Interactions
Hydrogen bonds are specific, short-range, and directional nonbonded interactions. They occur between a hydrogen atom bound covalently to an electronegative atom (usually N, S, or O) and an additional electronegative atom (Table 1). Distances of 2.5–3.2 Å between hydrogen-bond donor X and Y and X–H⋯Y angles of 130–180° are typically found. Their strength is optimal when the three concerned atoms are aligned and when the H donor tends to point directly at the acceptor electron pair. As a result of its electrostatic nature, the strength of a hydrogen bond depends also on its microscopic environment and on the local dielectric constant ε of the surrounding medium (Coulombic interaction energy is proportional to ε−1). Therefore, buried hydrogen bonds are regarded as more important for protein–ligand interactions than those formed in solvent-exposed regions. The free energy for hydrogen bonding can be between −4 and −30 kJ/mol, but usually is in the range of −12 to −20 kJ/mol. Binding affinities increase by about one order of magnitude per hydrogen bond. Although their strength is weaker than ionic or covalent bonds, they are in general the predominant contribution to the specificity of molecular recognition.
Table 1. Potential Hydrogen Bond Donor and Acceptor Groups Classified According to Their Strength of Interaction
| Donora | Acceptor | |
|---|---|---|
| Very strong | N+H3, X+–H, F–H | CO2-, O-, N-, F- |
| Strong | O–H, N–H, Hal–H | O=C, O–H, N, S=C, F–H, Hal- |
| Weak | C–H, S–H, P–H, M–H | C=C, Hal–C, π, S–H, M, Hal–M, Hal–H, Se |
a: X is any atom, Hal is any of the lighter halogens, and M is a transition metal.
